Quality Control |
| Our company is equipped with a full set of high-precision electromechanical testing equipment, backed by comprehensive quality management, rigorous and scientific design processes, and integrated electromechanical manufacturing techniques. Through systematic and standardized reliability verification, as well as precisely controlled operational procedures, we ensure that product quality is meticulously managed from the very beginning of the design phase. |

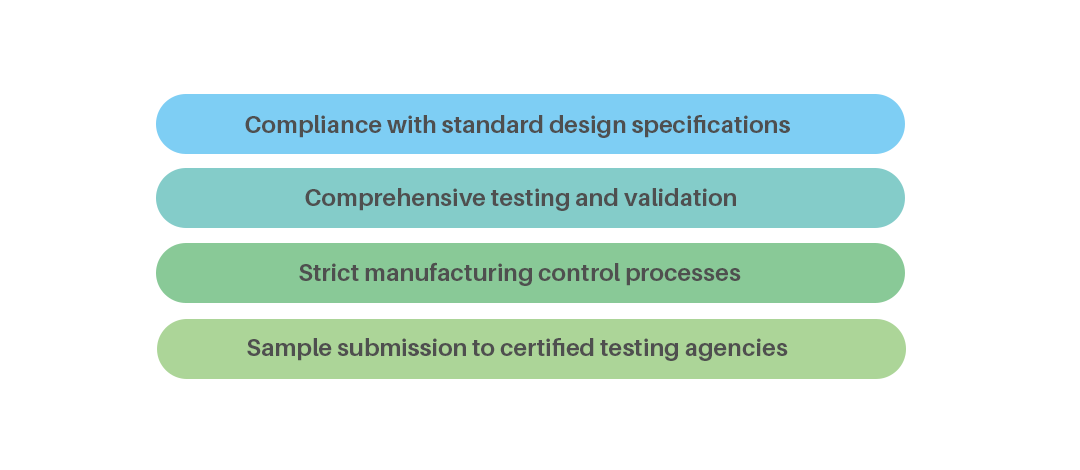
| To ensure product quality meets customer requirements, our company has implemented and achieved compliance with a series of internationally recognized quality management standards, including GMP 820, ISO 9001, and ISO 14001. Every product must undergo rigorous testing and validation. We simulate real-world usage conditions through stringent tests such as high/low temperature testing, drop and impact testing, and continuous thermal aging testing, ensuring superior performance and reliable quality. |